There’s something about the greenhouse effect /sky radiation / downwelling infrared / back radiation issue that keeps drawing me back to the subject.
There’s something about the greenhouse effect /sky radiation / downwelling infrared / back radiation issue that keeps drawing me back to the subject.
I guess it’s the number of people who don’t believe the so-called greenhouse effect exists (I still get e-mails from them, even today), combined with the difficulty of convincing them that their everyday experience is consistent with its existence.
I’ve used a handheld IR thermometer to directly measure its effect (the temperature of the surface of a thermopile in the device increases as you scan from pointing straight up in a clear sky to pointing at an angle…voila! Downwelling sky radiation changing surface temperature!). But, no, that’s not enough.
I’ve also tried to explain that the temperature of the Earth and its atmosphere (or anything else, for that matter) is a function of rates of energy gain and energy loss, not of just how much solar radiation is absorbed. I’ve argued this using the analogy of insulation in a house…even though the insulation does not add “new energy” (just as the atmospheric greenhouse effect doesn’t add new energy to the Earth system,) it does make the house warmer in winter by reducing the rate of energy loss to its colder surroundings.
But, no, that’s not accepted since, you know, house insulation works by conduction, not by radiation. Sigh.
So, just for laughs, here’s another demonstration, involving a simple model of the cooling of the soil at night.
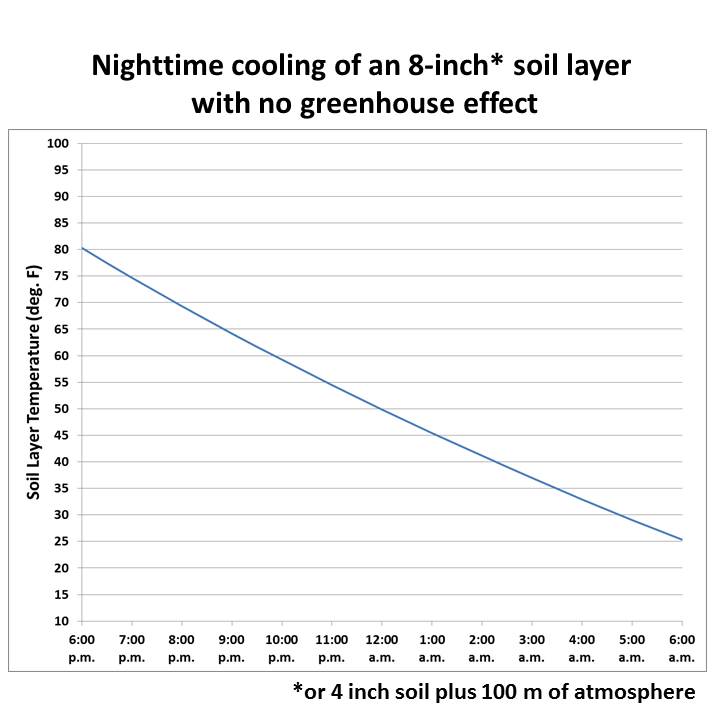
At night the soil cools by loss of infrared radiation. The Stefan-Boltzmann equation lets us estimate the rate at which IR energy is being lost based upon surface temperature and emissivity, and simply dividing that by the product of the soil depth and soil bulk heat capacity gives us the rate at which the soil layer temperature will fall. Basic physics and thermodynamics.
From that we can make a simple time-dependent model to calculate the change in temperature throughout the night. This simple spreadsheet model I’ve provided here will allow you to change assumed parameters to see how to get a realistic temperature decline over 12 nighttime hours. What you will find is that the temperature falls to unrealistically cold levels unless you assume a large downwelling energy flux from the sky into the soil (also adjustable in the model).
If you are wondering, “what about cooling of the atmosphere in contact with the ground?”, well just make the soil layer deeper…it turns out that 0.2 meters of soil is equivalent in bulk heat capacity to about 200 m of atmosphere.
The adjustable parameters (in red in the spreadsheet) are soil depth (0.2 m is typical for day-night temperature changes), the soil heat capacity (2.5 is typical, water is 4.18), the IR emissivity (0.90-0.95 would be typical), the downwelling sky radiation intensity (0 for all you sky dragon slayers [SDSs] out there, 250-350 for the rest of us), and the starting temperature (300 K is about 80 deg. F).
For example, for a 0.2 m moist soil layer (about 8 inches thick), starting at 80 deg. F, the rate of energy loss over 12 hours is enough to cool that soil layer down to 25 deg. F….IF you don’t assume any downwelling IR from the sky (the SDS-recommended setting):
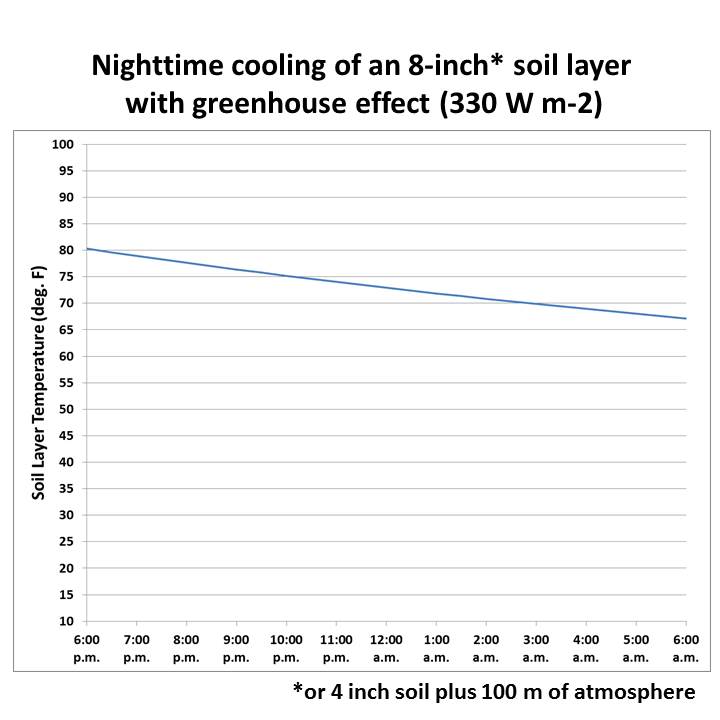
But, if you assume the Trenberthian global-average value of 330 W/m2 for downwelling sky radiation, the soil cools from 80 to about 67 deg. F, a much more realistic value:
In reality, the soil surface cools faster that does the deeper layers, but I didn’t want to complicate things with a multi-level model (which I don’t have time to work on anyway). This is just a simple, bulk model calculation meant to illustrate how extremely cold it would get at night without downwelling IR from the sky (aka, the “greenhouse effect”) reducing the net rate of energy loss to outer space.
Of course, downwelling IR from the sky is going on 24-7-365, acting to keep daytime temperatures warmer than they would otherwise be, too.
Warmer daytime + warmer nighttime = Warmer Earth.
Now, as I’ve mentioned before, as much as 75% of this big, bad greenhouse effect is “short-circuited” by convective heat loss by the surface, which is almost entirely a daytime phenomenon over land (nighttime surface temperatures quickly cool the near-surface air to make it convectively stable). (And, just for completeness, a greenhouse atmosphere is colder in the upper layers than it would otherwise be.) My point is that, just because the “greenhouse effect” exists doesn’t mean that our 1-2% enhancement of it with carbon dioxide is going to cause anything bad to happen. There are natural cooling mechanisms in place for the lower atmosphere. The stratosphere, though, probably will cool, as we have seen in satellite data.
But denying the existence of downwelling IR radiation from the sky (which is measured continuously at many sites around the world) is, in my opinion, a losing strategy.
Now, let the silliness begin….

 Home/Blog
Home/Blog