I continue to receive emails (not to mention the hundreds of sometimes nasty blog comments) objecting to what I just expressed in the title of this article. So, I thought it would be useful to propose a simple experiment that demonstrates the concept.
This is a considerably simpler task than my recently proposed experiment to measure the warming effect of adding CO2 to the atmosphere (which I now believe was not possible, at least as I originally proposed it). This experiment would be easy enough for high school students to perform, and maybe even junior high students. It probably does require a good multi-probe temperature monitoring and data logging device. I use the Extech SD200 three-probe temperature sensor. Alternatively, a $50 handheld IR thermometer might be used in a pinch, if you are careful.
Background
One of the supposed arguments against atmospheric greenhouse gases keeping the Earth’s surface warmer than if those gases were not present is the claim that, since the atmosphere is colder than the surface, it would violate the 2nd Law of Thermodynamics for a cold object (the atmosphere) to increase the temperature of a warm object (the Earths surface).
The Wikipedia entry for the 2nd Law of Thermodynamics includes the following statement from Rudolph Clausius, who formulated one of the necessary consequences of the 2nd Law (emphasis added):
“Heat can never pass from a colder to a warmer body without some other change, connected therewith, occurring at the same time.”
The statement by Clausius uses the concept of ‘passage of heat’. As is usual in thermodynamic discussions, this means ‘net transfer of energy as heat’, and does not refer to contributory transfers one way and the other.
The italicized words are important, and have been ignored by my critics: while it is true that the net flow of heat must be from higher temperature to lower temperature, this does not mean that the lower temperature object cannot (for example) emit radiant energy in the direction of the warmer object, and thus increase the temperature of the warmer object above what it would otherwise be.
But, this statement I just made will lead to endless arguments and objections (watch the comments, below), with hand waving qualitative statements about absorbing and emitting molecules and photons and entropy and perpetual motion and such.
So, let’s envision a simple experiment that will mimic what happens in the climate system, using visible light to heat a warm surface which then cools through infrared radiation toward a cold surface.
Energy Balance of the Global Climate System
The sun’s energy that is absorbed by the Earth’s surface raises its temperature. The surface then loses energy through both (1) non-radiative loss to the atmosphere (conduction, evaporation, and convection), as well as (2) infrared radiative loss to the atmosphere and to outer space.
What is interesting is that the clear atmosphere is mostly transparent to the sunlight passing through it and warming the system, but it is not transparent to the infrared radiation trying to escape back out to cool the system. This is the basis of the atmospheric “greenhouse effect”.
Now, in order for the climate system to maintain a roughly constant average temperature, there must be energy balance: the rate at which the earth-atmosphere system gains energy from the sun must match the rate at which the system loses infrared energy to the cold depths of outer space, which has a radiating temperature of almost absolute zero. If you can reduce the rate at which it cools to outer space, the climate system will increase its temperature until it emits enough infrared energy to restore radiative balance. This is the basis for global warming theory: increasing carbon dioxide in the atmosphere reduces the rate of IR energy loss to deep space, resulting in some warming. (The warming is actually in the lower atmosphere, while the upper atmosphere cools).
The Experiment
We can mimic these radiative processes by continuously heating one surface with halogen light bulbs (which more closely approximates the solar spectrum of light than incandescent bulbs). The hot surface will then radiatively cool toward a second surface which is chilled (e.g. with dry ice inside a cooler). The heated surface will also lose heat through conduction to the surrounding air, too, but we will reduce that effect with Styrofoam insulation….what we are looking for is a radiative effect.
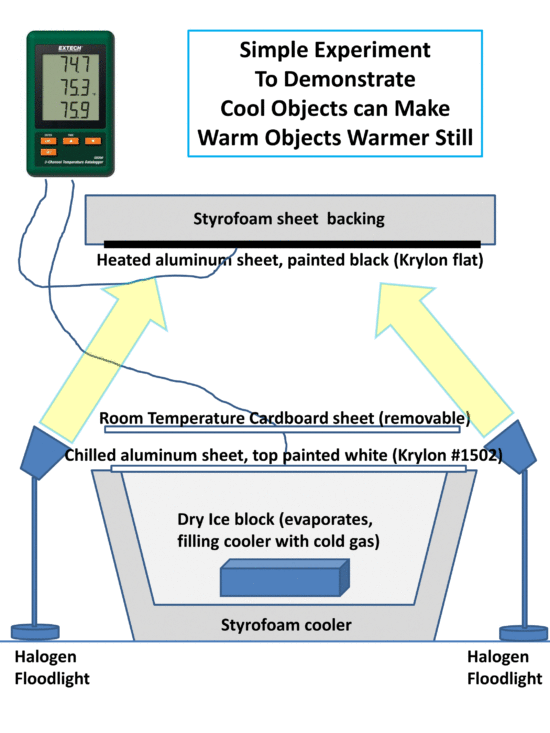
The following cartoon shows the basic setup.
The heated surface is painted black to absorb as much visible light as possible and raise its temperature. The chilled surface is painted with Krylon white #1502 which has an infrared emissivity close to 1.0 (allowing it to efficiently absorb IR energy from the heated surface) while the white color also reflects visible light and so avoids heating from the lamps.
At some point, energy balance will be reached when temperatures stabilize. (Of course, eventually all of the dry ice will be used up…so there is limited time to do the experiment…maybe an hour or two). I suggest putting the heated surface on top so any heated air goes upward and away from the experimental setup. Similarly, the chilled surface will have chilled air spilling down the sides.
Now, if we simply insert a piece of room-temperature cardboard in between the heated surface and the chilled surface, we should see an increase in the temperature of the heated surface despite the fact that we just used a cooler (room temperature) object to make a warmer object even warmer still, in apparent violation of the 2nd Law of Thermodynamics. The cardboard can probably just be laid on top of the cooler. Or, a sheet of Styrofoam might work just as well, if not better. The temperature of the air between the heated and chilled surfaces could be monitored with the third probe from the SD200 to answer any objections that the intervening cardboard is somehow reducing the mixing of air between the hot and cold surfaces (which shouldn’t happen anyway, if the heated surface is above and the chilled surface is below).
The intervening cardboard (or Styrofoam) sheet mimics what the atmospheric greenhouse effect does, at least in terms of energy flows (but it’s a solid surface, rather than a gas, so maybe it’s more analogous to the greenhouse effect of thick cirrus clouds, which completely block the transfer of infrared light).
I don’t know just how much the observed temperature increase in the heated surface will be when the cardboard sheet blocks its view of the chilled surface. Maybe 1 deg. F, maybe 10 deg. F. But it should be observable. The effect will be greater the bigger the temperature difference that can be maintained between the heated and chilled surfaces, and the closer you can get them together so the heated surface “sees” mostly the chilled surface, instead ot the room-temperature surroundings with which it is also exchanging infrared radiation.
Now, this experiment does not prove that gases can do what the cardboard has done; that is a separate issue that is much more complicated to demonstrate with an experimental setup. It only answers the 2nd Law violation claims some have made against a cool object (here, the cardboard sheet) causing a heated object to be warmer than if the cool object was not present, which is what the Earth’s greenhouse effect does.
NOTE TO COMMENTERS: I intend to delete any comments which include personal insults.
NOTE TO READERS OF COMMENTS: Some commenters here throw around technical terms and make grand assertions and detailed arguments which I consider fallacious. I do not have time to counter them all every time they arise, although I have addressed virtually all of them in other posts over the years.

 Home/Blog
Home/Blog