Measuring The (Nonexistent) Greenhouse Effect in My Backyard with a Handheld IR Thermometer and The Box
Laypersons are no doubt confused by all of our recent esoteric discussions regarding radiative transfer, and whether global warming is even possible from a theoretical standpoint.
So, let’s take a break and return to the real world, and the experiments you can do yourself to see evidence of the “greenhouse effect”.
One of the claims of greenhouse and global warming theory that many people find hard to grasp is that there is a large flow of infrared radiation downward from the sky which keeps the surface warmer than it would otherwise be.
Particularly difficult to grasp is the concept of adding a greenhouse gas to a COLD atmosphere, and that causing a temperature increase at the surface of the Earth, which is already WARM. This, of course, is what is expected to happen from adding more carbon dioixde to the atmosphere: “global warming”.
Well, it is one of the marvels of our electronic age that you can buy a very sensitive handheld IR thermometer for only $50 and observe the effect for yourself.
These devices use a thermopile, which is an electronic component that measures a voltage which is proportional to the temperature difference across the thermopile.
If you point the device at something hot, the higher-intensity IR radiation heats up the hot-viewing side of the thermopile, and the IR thermometer displays the temperature it is radiating at (assuming some emissivity…my inexpensive unit is fixed at e=0.95).
If you instead point it at the cold sky, the sky-viewing side of the thermopile loses IR radiation, cooling it to a lower temperature than the inside of the thermopile.

For instance, last night I drove around pointing this thing straight up though my sunroof at a cloud-free sky. I live in hilly territory, the ambient air temperature was about 81 F, and at my house (an elevation of 1,000 feet), I was reading about 34 deg. F for an effective sky temperature.
If the device was perfectly calibrated, and there was NO greenhouse effect, it would measure an effective sky temperature near absolute zero (-460 deg. F) rather than +34 deg. F, and nighttime cooling of the surface would have been so strong that everything would be frozen by morning. Not very likely in Alabama in August.
What was amazing was that driving down in elevation from my house caused the sky temperature reading to increase by about 3 deg. F for a 300 foot drop in elevation. My car thermometer was showing virtually no change. This pattern was repeated as I went up and down hills.
The IR thermometer was measuring different strengths of the greenhouse effect, by definition the warming of a surface by downward IR emission by greenhouse gases in the sky. This reduces the rate of cooling of the Earth’s surface (and lower atmosphere) to space, and makes the surface warmer than it otherwise would be.
If you have a day where there are patches of blue and clouds, you can point the thermometer at the clouds and pick up a warmer reading than the surrounding blue sky.
I did it this morning (see photo, above). When I moved from a view of the blue sky to the patch of clouds, the sky-viewing side of the thermopile became warmer…even though the thermopile is already at a higher temperature than the sky. The display would read a few degrees warmer than the reading looking at blue sky.
If you perform this experiment yourself, you need to be careful about the elevation angle above the horizon you are pointing being about the same. Even in a clear sky, as you move from the zenith (overhead), down toward the horizon the path length of sky the IR thermometer sees increases, and so you measure radiation from lower altitudes, which are warmer. This makes the effective sky temperature goes up. (This is ALSO evidence of the greenhouse effect, since looking at the sky above the horizon is like adding greenhouse gases to the atmosphere overhead. The (apparent) concentration of greenhouse gases in the lower atmosphere goes up, and so does the intensity of the back radiation.)
Even earlier in the morning, about 5:30, the middle-level clouds were thicker, and I measured a sky temperature in the 50’s F. We will see more evidence of that using air temperatures, below.
This shows that the addition of an IR absorber/emitter, even at a cold temperature (the middle level clouds were probably somewhere around 30 deg. F), causes a warm object (the thermopile) to warm even more! This is the effect that some people claim is impossible.
Remember, the IR thermometer calibrated temperature output is based upon real temperatures, the temperatures on either side of the thermopile.
And if you think this is just an effect of some sunlight reflecting off the cloud….read on.
Evidence from The Box
I have been seeing the same effect in “The Box”, which is my attempt to use the greenhouse effect to warm and cool a thin aluminum plate coated with high-emissivity paint, that is heavily insulated from its surroundings in order to isolate just the radiative transfers of energy between the sky and the plate. This can be considered a clumsy, inefficient version of the IR thermometer. But now, *I* am making actual temperature measurements.
The following plot (click on it for the full-size version) shows data from the last 2 days, up through this morning’s events. The plate gets colder at night than the ambient temperature because it “sees” the cold sky, and is insulated from heat flow from the surrounding air and ground.
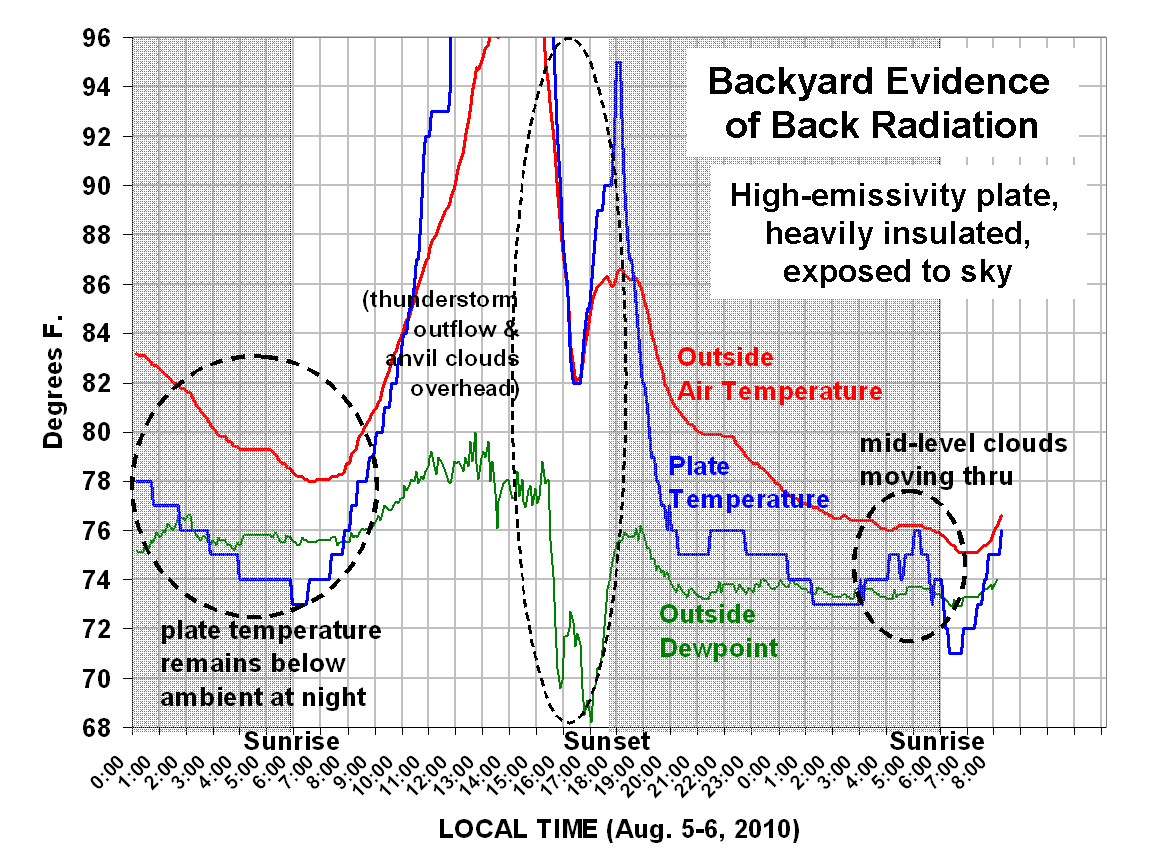
In the lower right, I have also circled where thin middle-level clouds came over, emitting more IR radiation downward than the clear sky, and causing a warming of the plate. Since the plate is mostly isolated from heat exchanges with the surrounding air and warm ground, it responds faster than the ambient air temperature to the intensity of “back radiation” downwelling from the sky.
When I woke this morning before sunrise, around 5:30, I saw these mid-level clouds (I used to be a certified aviation weather observer), I measured about 50 deg. F from the handheld IR thermometer.
This supports what people already experience…cloudy nights are, on average, warmer than clear nights. The main reason is that clouds emit more IR downward, change the (im)balance between upwelling and downwelling IR, and if you change the balance between energy flows in and out of an object, its temperature will change. Conservation of Energy, they call it.
(WARNING: a technical detail about the above measurements and their importance to greenhouse theory follows.)
What this Means for the Miskolczi “Aa=Ed” Controversy
Except for relatively rare special cases, the total amount of IR energy downwelling from the sky (Ed) will ALWAYS remain less than the amount upwelling from below and absorbed by the sky (Aa). As long as (1) the atmosphere has some transparency to IR radiation (which it does), and (2) the atmosphere is colder than the surface (which it is), then Ed will be less than Aa…even though they are usually close to one another, since temperatures are always adjusting to minimize IR flux divergences and convergences.
But it is those small differences that continuously “drive” the greenhouse effect.
 |

 Home/Blog
Home/Blog



