And, yes, you can try this at home.
I put together a simple surface energy balance model in an Excel spreadsheet so people can play around with the inputs. It computes the time changing surface temperature for any combination of:
1) absorbed sunlight (nominally 161 W/m2)
2) ocean mixed layer depth (does not affect final equilibrium temperature)
3) initial temperature of the ocean mixed layer (does not affect final equilibrium temperature)
4) atmospheric IR transmittance (yes, you can set it to 1 if you are carrying your sky dragon slayer [SDS] ID card)
5) effective temperature of downwelling sky radiation (nominally 283 K, but in effect becomes zero if transmittance=1)
6) surface convective heat loss (nominally 97 W/m2)
The nominal values are set to be consistent with the Kiehl-Trenberth global energy budget diagram.
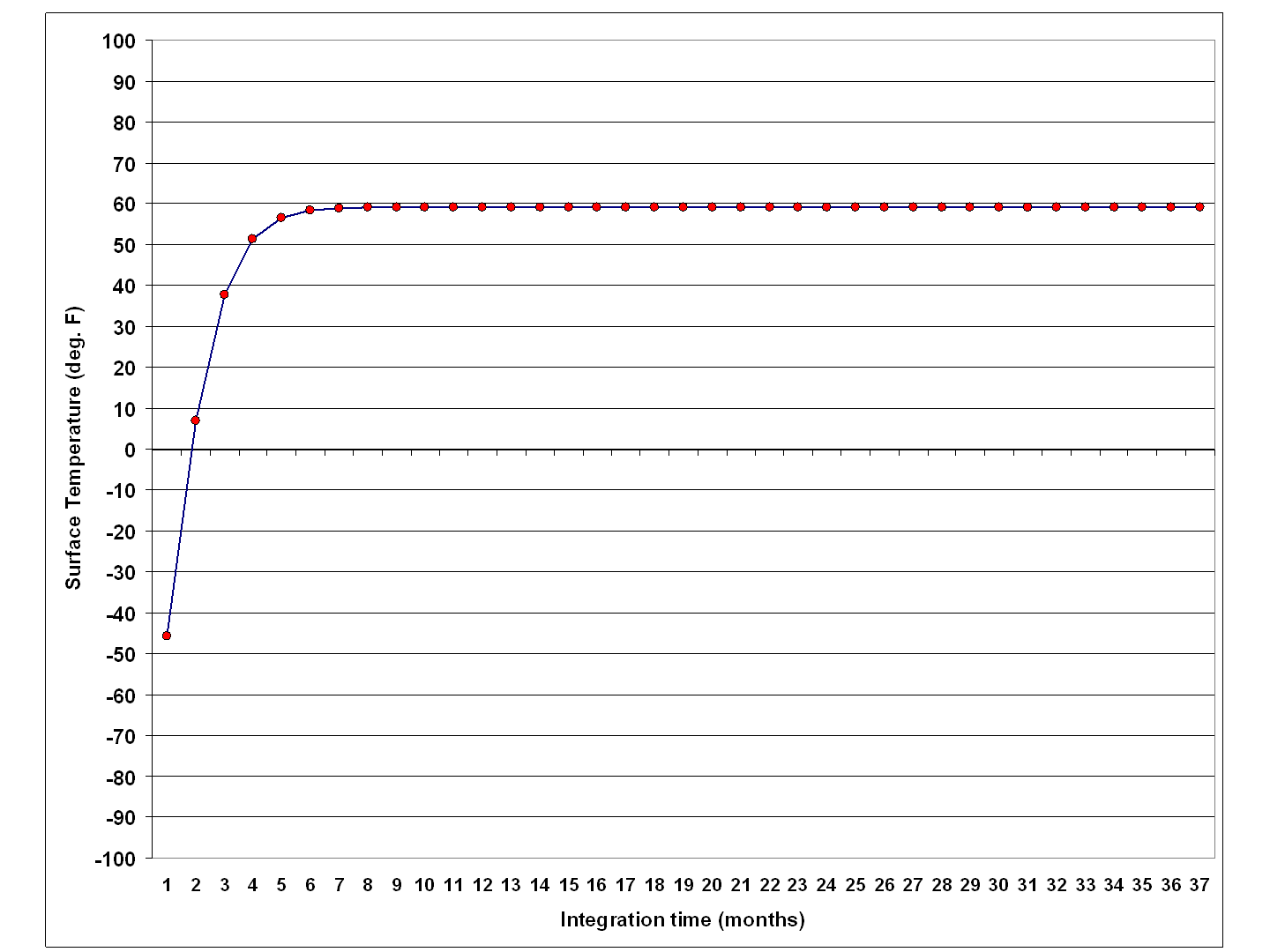
The simple model is good for developing intuition about how the equilibrium surface temperature changes when varying different input parameters. I would challenge people to see what other combinations of parameters result in the observed global average surface temperature, which is believed to be around 59 deg. F or so.
Again, the model is very simple; it changes the temperature of the assumed ocean mixed layer depending upon assumed rates of energy gain and energy loss by that layer.
And here is the model: simple-sfc-model
Example: Nighttime cooling with and without the greenhouse effect
There’s an interesting experiment you can run with the model to see how nighttime temperatures cool off depending upon whether there is a greenhouse effect or not. If you run the model starting the surface temperature around 288 K (the observed global average), turn solar absorption off (nighttime), make the time step 1/24 of a day (1 hour), and make the ocean mixed layer approximate a land surface (set depth to 0.1 meter), you will find that the surface cools about 20 deg F overnight when the atmospheric IR transmittance is set to 0.1 (realistic), but cools by about 70 deg. F if the transmittance is set to 1 (no greenhouse effect). In other words, large amounts of atmospheric “back radiation” (admittedly a poor term) are required to explain why nighttime temperatures do not cool off more than observed.
PLEASE keep the discussion about gaining insight from the simple model…I know all about its shortcomings.

 Home/Blog
Home/Blog