The standard explanation of the “greenhouse effect” is that it keeps the surface of the Earth warmer than it would otherwise be, through infrared radiation downwelling from the atmosphere. Even though this IR radiation is being emitted at a lower temperature than the surface, it actually keeps the surface warmer. Some people have trouble with this explanation, claiming it violates one or more laws of thermodynamics.
As I have discussed ad nauseum, the temperature of a heated object is always determined by rates of energy gain and energy loss, and that energy loss is almost always a function of the object’s cooler surroundings.
Whether one views the greenhouse effect as extra infrared energy gained by the surface from the cooler atmosphere, or just a reduced rate of infrared energy loss by the surface to the atmosphere and outer space, the effect is the same: a surface temperature increase.
I’ve been toying with a few different ways to demonstrate this effect with a simple experimental setup using household items. Apparently the IR thermal imager, which I showed directly measures the surface temperature effects of varying levels of downwelling IR sky radiation on a microbolometer within the instrument, is not sufficient for some people.
So, I’ve come up with the following simple setup, and if I carry it out, I want predictions from readers here of what will happen to the temperatures of the 2 heated metal plates:
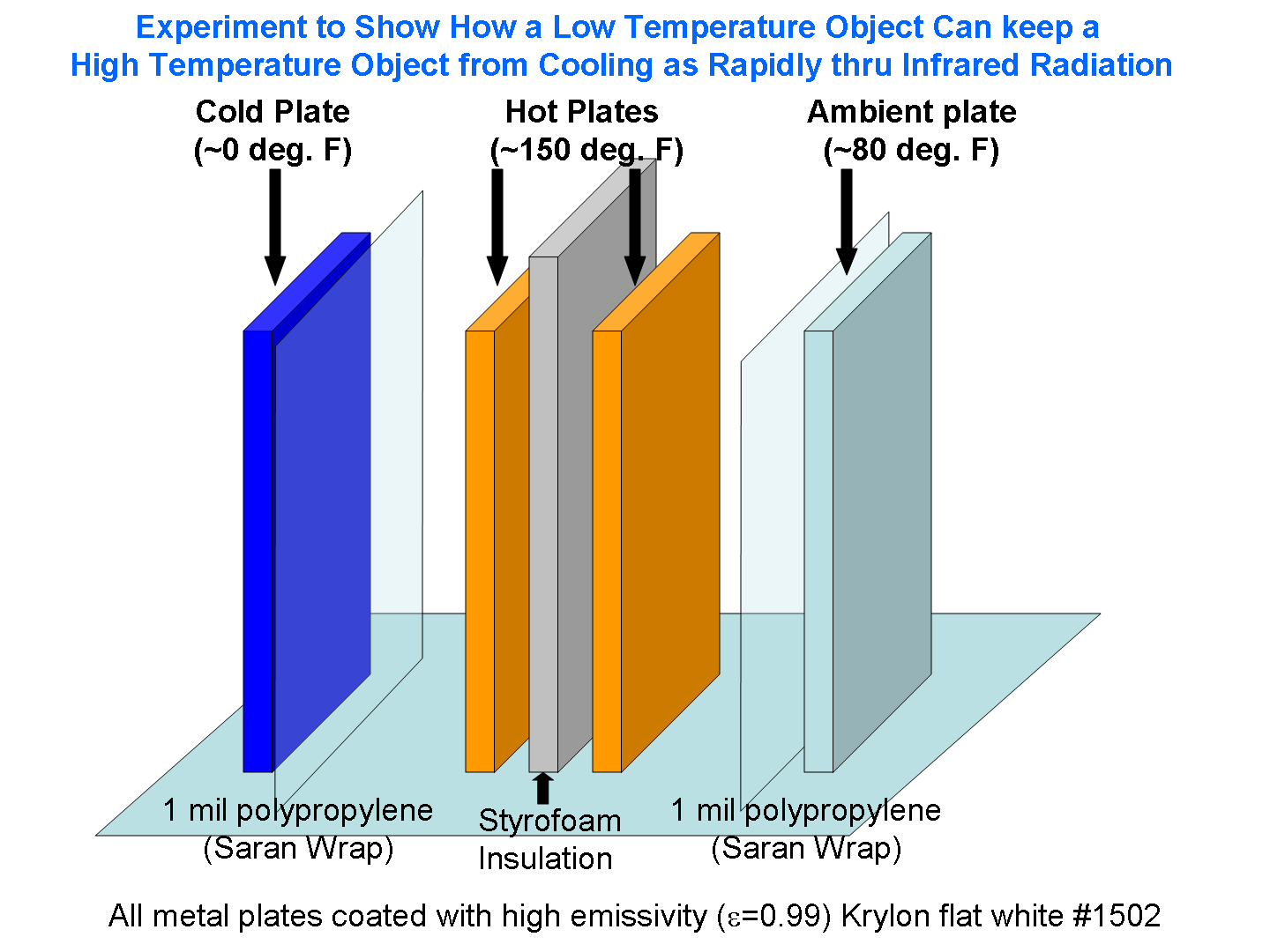
The two metal plates will be heated in the oven to the same temperature, then placed vertically next to each other, but separated by a sheet of Styrofoam. Obviously, the plates will cool, partly by conduction to the surrounding air. The above cartoon is just a rough approximation of the setup. I will probably have the ends of the heated plates covered by Styrofoam as well, to help reduce conductive heat loss.
But the plates also cool from infrared energy loss. So, I will expose one of the heated plates to a third plate that I will have chilled to at least 0 deg. F in the deep freeze.
Finally, I will expose the other heated plate to a 4th plate just at the ambient air temperature, say 80 deg. F.
Very thin sheets of polypropylene (Saran wrap), which are nearly transparent to IR radiation, will be used to minimize the movement of air currents between the heated plates and their cooler counterparts. All 4 plates will be coated with high emissivity (0.99) Krylon flat white #1502 paint.
My question is this: Will the two hot plates cool at different rates? I predict the heated plate exposed to the ambient (80 deg. F) plate will consistently stay warmer than the other heated plate exposed to the chilled (0 deg. F) plate.
Of course, if one waits long enough, all plates will come to the same temperature, since the hot plates are not actively heated (like the climate system is by the Sun) and the cold plate is not actively chilled (which would partly mimic the infrared energy sink of deep space).
The main point is that cooler objects which surround heated objects affect the heated objects temperature. As far as I can tell, this is a universal truth, with examples all around you. I find it mind boggling that some people do not accept it. (For anyone tempted to say, “But a cooler star doesn’t make a hotter star hotter still”, stay tuned for an experiment Anthony Watts has been working on).
I will monitor the plates’ temperatures with my FLIR i7 thermal imager. Because there is still a small amount of reflection from the heated plates (0.01) the thermal imager must be pointed at an angle which will not pick up reflection from the cooler plates, which would bias the results. Another option would be to buy 2 inexpensive car thermometers with a remote display.
Again, I want to hear some predictions: Will the hot plates cool at different rates? If so, do you see a mechanism other than infrared energy transfer which will explain the different rates of cooling?
If you see pitfalls in the experimental setup, then feel free to point them out and suggest how to mitigate them.
UPDATE: I will be periodically checking in and deleting comments which do not directly address the above experiment and what results it will produce…unfortunately, the comments are already getting sidetracked.

 Home/Blog
Home/Blog



