
One of the oft-cited objections to the term “greenhouse effect” is that it is a misnomer, that a real greenhouse (you know, the kind you grow plants in) doesn’t work by inhibiting infrared energy loss. It is usually claimed that a real greenhouse works by inhibiting convective heat loss by trapping the sun-heated air inside.
While working on a new website devoted to answering greenhouse questions, I decided to examine this issue. What piqued my interest was a couple quick back-of-the-envelope calculations that (1) for a glass covered greenhouse, the downward infrared (IR) emission from the roof should be about 100 W/m2 more than from a clear sky (a pane of glass is high emissivity, and opaque to broadband infrared), and (2) the realization that a greenhouse generates its own convection from the roof because the glass heats up, so convective air currents inside have their heat conducted (albeit inefficiently) through the glass, then the warm glass of the roof causes its own convection.
Add to this the fact that greenhouses are usually vented, which means they lose heat convectively anyway that by-passes the greenhouse structure.
So, the question is: Does a greenhouse work more from infrared heating (the “greenhouse effect”), or more from the inhibition of convective heat loss?
First, let’s examine some approximate energy fluxes for vegetation in the summertime. These are only rough estimates, and there are rather large variations in these depending on cloud cover, etc., and to be meaningful they need to represent a day+night average (infrared fluxes are orange; solar are yellow; convective are blue):
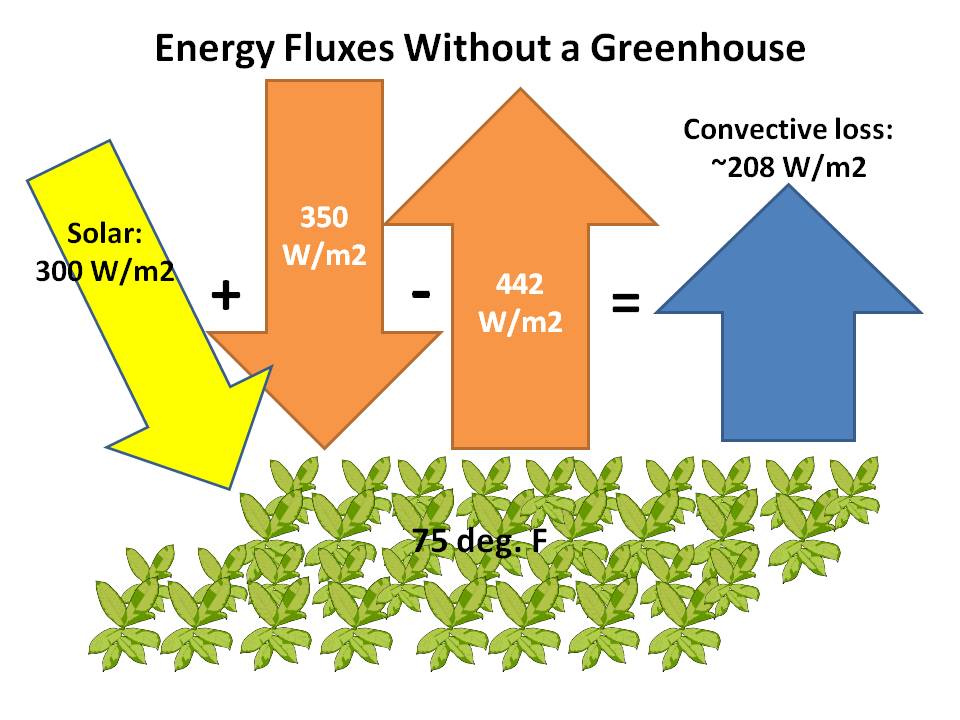
For simplicity, the calculated IR emission from solid surfaces assumes an emissivity of 1. The downward sky infrared is consistent with the BSRN network measurements during the warm season. Note that the energy fluxes have to sum to zero for temperature equilibrium, and we will ignore the photosynthetic storage of energy in plants which is very inefficient.
Now, with a greenhouse in place, we assume the average temperature of the interior rises, and that the glass roof reaches a temperature intermediate between the inside and outside air temperatures:
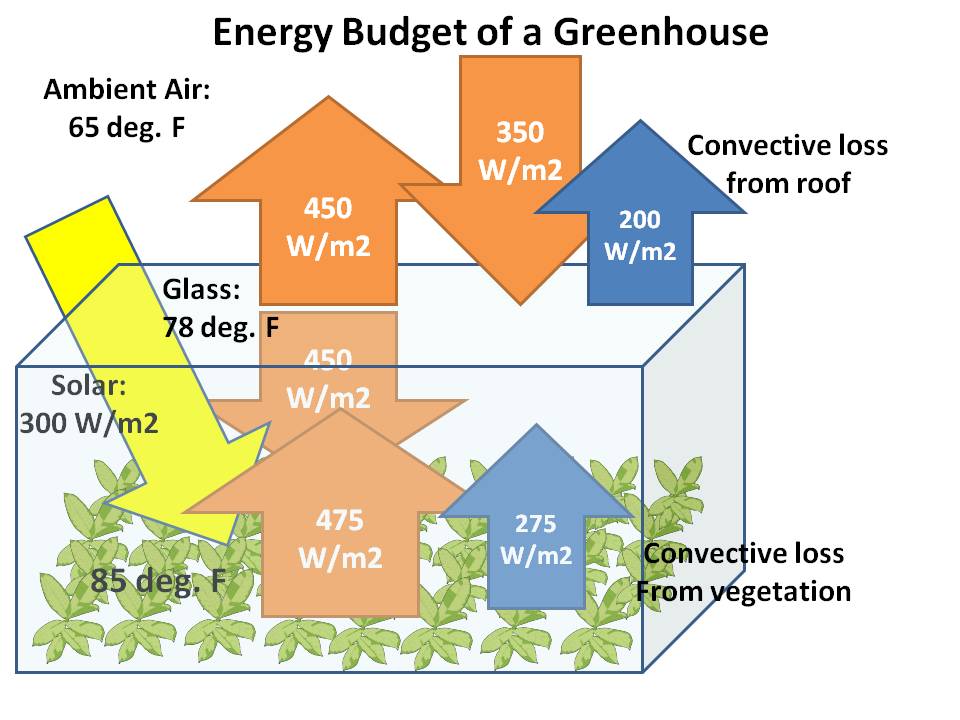
What really changes a lot is the downwelling IR, increasing from the sky value of 350 W/m2 to 450 W/m2, an increase of 100 W/m2. Convective heat generated (but temporarily “trapped”) within the greenhouse increases substantially, from 208 without the roof to 275 with the roof, for an increase of 67, which further heats the air, which in turn is helping to heat up the roof.
But notice that the convective heat loss by the greenhouse roof (200 W/m2, inferred as a residual) is only 8 W/m2 less than if the greenhouse was not there (208 W/m2). In contrast, the extra IR energy “input” (actually, reduced IR “loss”) is twelve times as large (100 W/m2) as the reduction in the convective loss (8 W/m2).
Of course, changing any of the assumed numbers will change the result. But, assuming I haven’t made a fundamental mistake, I think you would find that the “greenhouse effect” will consistently be larger than the convective inhibition effect.
So, maybe the greenhouse effect really does work like a real greenhouse. Again, the basic issue is this: replacing the downwelling sky radiation with a roof that is opaque to infrared (but still transparent to sunlight) represents a huge decrease in the IR energy loss by the vegetation, whereas the greenhouse roof still generates convective heat loss nearly as large as if the greenhouse wasn’t there.
I’m open to ideas, and better estimates of energy fluxes on this subject. The problem is actually surprisingly difficult one to think through. There are many energy fluxes involved (I haven’t even addressed energy losses out the side of the greenhouse) and the trick is to know which are the important ones and which ones can be ignored for the purposes of a rough estimate.
For example, the emissivity of glass is less than 1, but what that means is that it “traps” even more IR energy inside because it partly reflects the higher levels of IR the warmer vegetation is emitting upward.
What do the experts say about all this? I’m sure this problem has been analyzed before, probably in great detail, by multiple aggie graduates in their theses. Unfortunately, a Google search on “greenhouses” and “energy budget” is hopelessly cluttered with pages related to the Earth’s greenhouse effect (wow! how did that happen?)
If anyone is aware of studies done on the energy budget of greenhouses (of the agricultural kind), I would appreciate a reference or two. But until someone finds a serious error in the above analysis, I’d say we might need to admit that the “greenhouse effect” is pretty accurately named.
UPDATE: It appears the debate was brought up in the literature by R. Lee (“The Greenhouse Effect”, J. Appl. Meteorology, 1973) who has been referenced by many as showing a greenhouse does not work through the greenhouse effect, but he curiously admits the analogy “is correct only with respect to the glass, not with respect to the space enclosed”. Well, duh. That’s the point…the glass produces a greenhouse effect. In any event, his paper was refuted by Edwin Berry (Comments on “The Greenhouse Effect”, J. Appl. Meteorology, 1974) who showed several problems with Lee’s analysis.
So, I guess I’m left wondering…where did the oft-cited claim that a greenhouse does not operate through a greenhouse effect come from?

 Home/Blog
Home/Blog




A poorly ventilated Attic can exceed 160 degrees F. No glass. Similarly, greenhouse heating results mostly by impeding the natural convection.
FROM The Physics of Atmospheres, Houghton: “Although known as the ‘greenhouse effect’ it might be noted that in practice it only provides a minor contribution to the warmth of a greenhouse that is largely due to the suppression of air motions within it.”
The greenhouse effect is purely radiative, involving energy input from the sun, and energy loss by infrared radiation, in the absence of convective heat loss. So, “greenhouse effect” is actually a good description of how a real greenhouse works. -Roy