So, TRMM is “coming home” after a very successful mission measuring tropical rain systems for over 17 years.
Back when the TRMM concept was being pitched by NASA-Goddard scientists at HQ, I was pitching the competing mission representing NASA-Marshall. In retrospect, John Theon (the Program Manager at the time) made the right decision and gave the go ahead to develop TRMM.
I helped campaign for the design of the TRMM Microwave Imager (TMI), but by the early 1990s our global temperature monitoring work was taking up most of my time and to everyone’s surprise (since my original expertise was rainfall measurement from satellites), I chose not to be part of the TRMM Team.
TRMM also carried one of the CERES Earth radiation budget instrument packages, which allowed researchers to document the diurnal cycle in cloud effects on reflected sunlight since TRMM was placed in a non-sun-synchronous orbit, as well as the Lightning Imaging Sensor (LIS) which was developed here in Huntsville, and a Visible and InfraRed Scanner (VIRS).
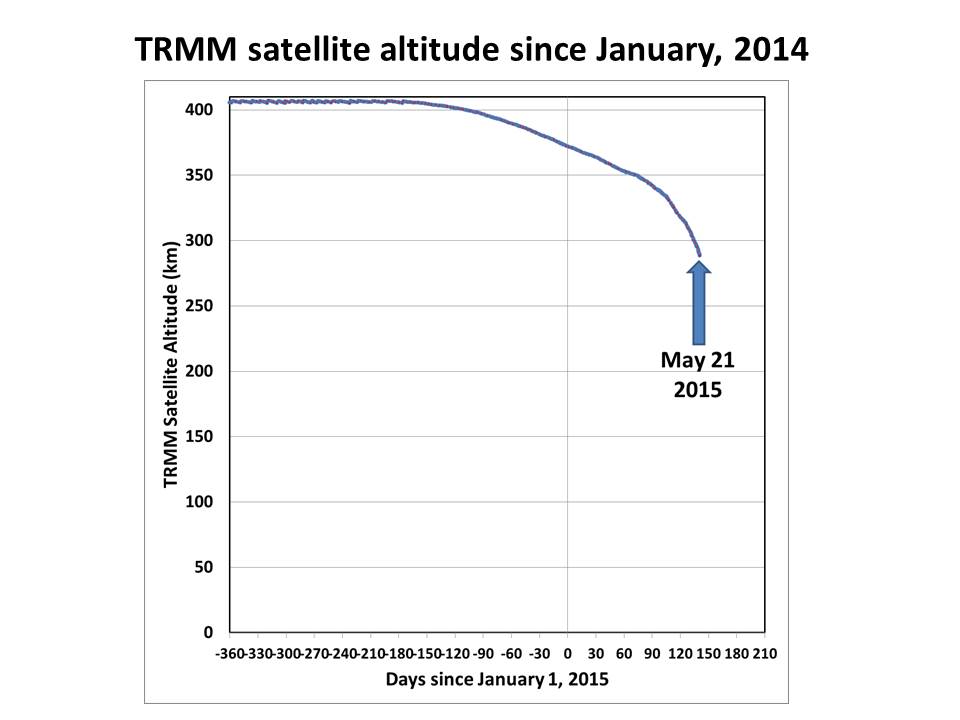
I’ve been tracking the fall of the TRMM satellite, and as can be seen it is now descending rather rapidly:
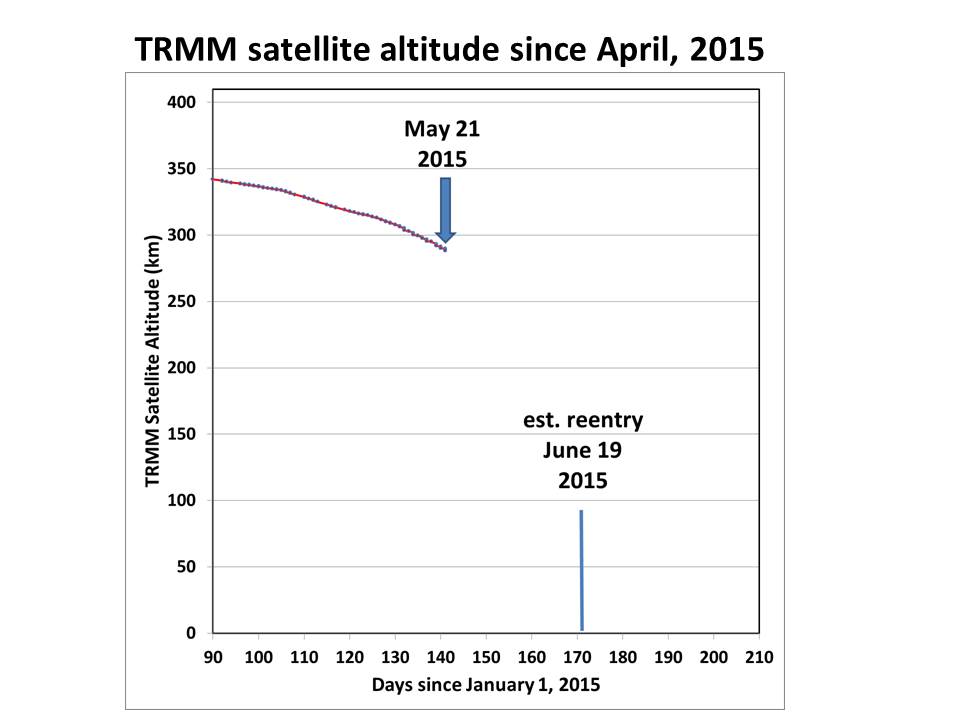
If we zoom in, we get a better idea of it’s trajectory in the last couple months, and Space-Track.org is now calculating a reentry date of June 19:
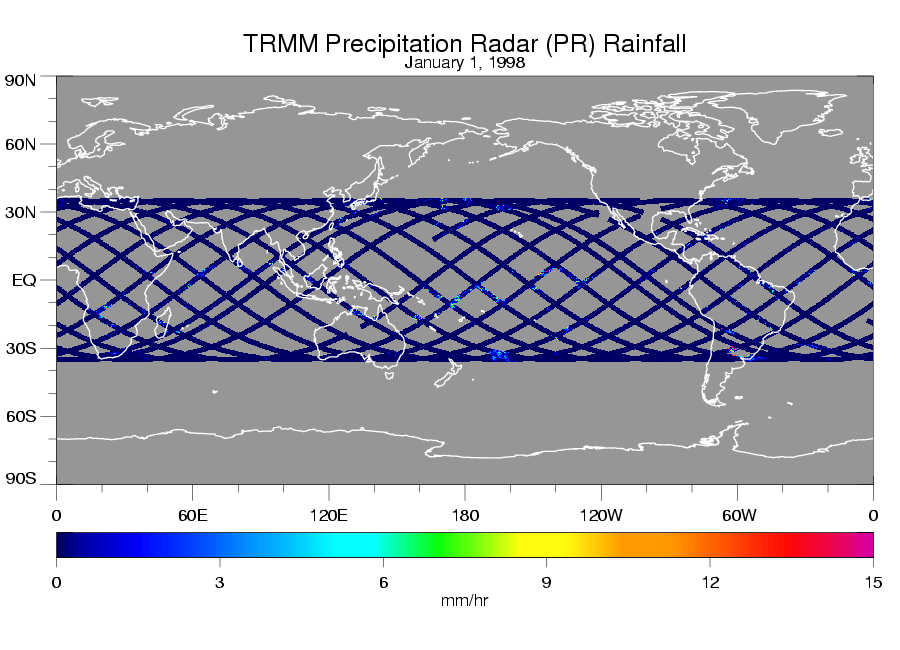
Once the satellite reaches about 90 km altitude, it reenters very quickly. Because the rate of descent is nonlinear, and depends upon the satellite orientation, which might be tumbling and causing variable amounts of atmospheric drag, it is almost impossible to determine where the satellite will fall…it could be anywhere between 35N and 35S latitude, as suggested by this single day of TRMM radar coverage:
The June 19 date could also change substantially…it might be many days off. For example, in just one day, the reentry date was moved up by a day by the Space-Track folks.
I’d like to congratulate all of the many engineers and scientists here in the U.S., in Japan (which provided the radar), and throughout the world, who made the TRMM mission such a great success.

 Home/Blog
Home/Blog