(UPDATED: 3:10 p.m. July 29, 2010 with temperature difference plot)
As promised, here are the first results from my little backyard experiment to investigate the role of downwelling infrared (IR) sky radiation on air temperature. (High school students looking for a science experiment, pay attention).
It’s a heavily insulated box that — theoretically — should chill air at night to a temperature below that of the outside air. The following is a conceptual design of The Box before I built it, along with the key components:
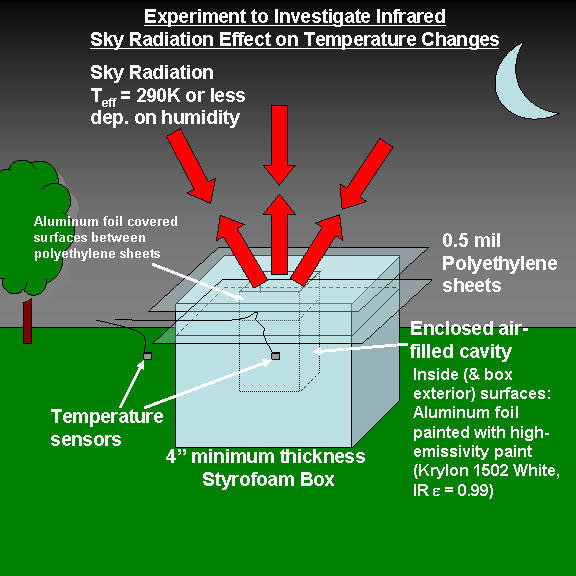
This all came about because I got tired of being asked about the theory behind global warming, specifically, how can downwelling infrared sky radiation from greenhouse gases (mostly water vapor, to a lesser extent CO2) cause global warming of the Earth surface, when the emitting temperature of the sky is colder than the surface?
Some people are convinced that this cannot happen, since the 2nd Law of Thermodynamics says energy naturally flows from higher temperature to lower temperature. In contrast, the mainstream science community, while agreeing the NET energy flow is from warm to cold, you can still cause warming by adding more greenhouse gas to the colder atmosphere. This happens even though the IR emitting temperature of the sky “causing” that warming is 10’s of degrees colder than the surface.
[NOTE: the direct warming effect of more atmospheric CO2 is small; its the resulting indirect warming (positive feedbacks) from clouds and water vapor that has most scientists worried. But not me…I think the net feedbacks are negative.]
The Box
So, since I have two automated weather stations in my backyard, I decided to build a heavily insulated box that would contain a small amount of air, and try to reduce all the other kinds of energy exchange between that air sample and the environment to a minimum EXCEPT for the influence of the downwelling sky radiation.
The air sample and the sky would be allowed to exchange IR radiation, and the colder the infrared emitting temperature of the sky is, the colder the air in the box should become compared to the air outside of the box. More about that later.
While we might not put the debate to rest with such an experiment, we can build some intuition about the energy flows that cause day and night air temperatures to be what they are. Of course, one could simply buy a hand-held infrared radiometer and take the sky’s “temperature” directly. But since everyone (myself included) has at least some trouble conceptualizing the role of infrared radiation in weather and climate (after all, we can’t see IR radiation), I thought that letting the IR effect be measured through its influence on temperature would make a bigger impact.
So, here’s a picture of the real thing that I took this morning, after collecting data since about noon yesterday:

The wireless data processor for the cavity temperature data is the little unit on the top. It sends a new temperature measurement every 5 minutes to my desktop computer in the house.
Here’s a close-up of the cavity. There is an insulating layer of air trapped between the two thin sheets of polyethylene, which are nearly transparent to infrared energy. The temperature sensor itself can be seen below that, in the cavity, the walls of which are painted with high emissivity paint (Krylon 1502 Flat White, IR emissivity = 0.99; Note that in the infrared, black is not necessarily more emissive than white…it depends on what the paint is made of, and whether the surface is rough or smooth).

Meanwhile, my regular weather station is about 20 feet away, and it is collecting air temperature and dewpoint data on the same schedule as The Box cavity temperatures are taken:

First Data from The Box
The first 17 hours of data, from midday yesterday until 8:05 a.m. this morning, are plotted below:
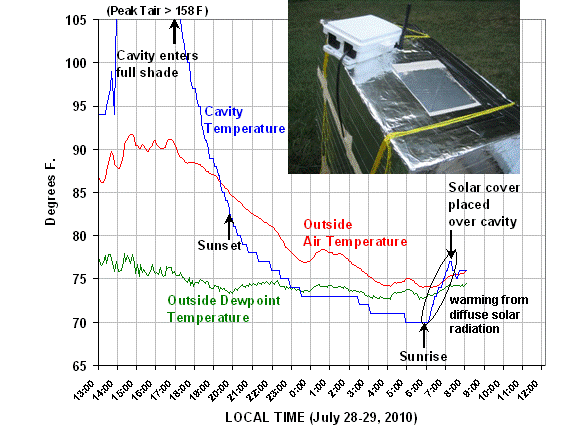
When I first closed up the box with the thermometer placed in the cavity, I was surprised how hot the cavity became. The maximum temperature recorded yesterday afternoon was 158 deg. F, and that must have been the limit for the sensor, because the temperature then flatlined for about an hour.
The reason for the high temperature was some direct sunlight reflecting off of one wall of the airspace, above the cavity. Even though the cavity was painted white, it still absorbed enough energy to make the air very hot. From what I have been able to gather, it is very difficult to get the solar reflectance of white paint above about 0.9.
It is interesting to calculate what rate of energy input would be required to cause this rapid rate of warming, which was about 3 deg. F per minute. If the cavity is initially in energy equilibrium, and we start reflecting 20 Watts per sq. meter more onto the cavity walls, about 10% of that (2 Watts per sq. meter) would be heating the paint, and so the air in the cavity.
According to my calculations, that would be more than enough to explain the initial rapid rise of temperature in the cavity on its way to 158+ deg. F. My calculations are only approximate, though, since I did not take into account the heat capacity of the cavity walls (painted aluminum foil), or the increased loss of IR as the cavity warmed, or conductive losses to the styrofoam and air space above the cavity.
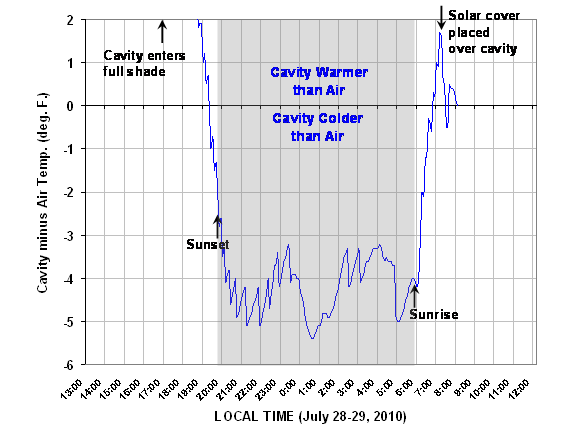
But what we are really interested in is what happens when the overwhelming influence of solar radiation subsides. In the above plot, look at what happens as sunset approaches. Despite diffuse solar radiation still entering The Box from the blue sky, the cavity air cools to a couple of degrees below the ambient air temperature by sunset. Then, during the night, the cavity air averages about 4 deg. F colder than the outside air. This is easier to see in the next plot of the temperature difference between the cavity and the outside air, which we see remains pretty constant during the night:
To see how even a little diffuse sunlight from the sky can cause warming of the cavity, note what happened just after sunrise this morning…even though our yard does not see direct sunlight till close to 11 a.m. (very tall trees in the way), the blue sky started warming the cavity almost immediately after sunrise.
Then, after a short while, I put a white cover from a plastic cooler over the cavity to minimize the daytime heating of the cavity. At the end of the data plot you can see this solar cover caused the cavity to cool back down to the same temperature as the ambient air.
So, we already can see the cooling effect of infrared radiation in the data…in the form of cavity temperatures colder than the air. This happens from just before sunset, until sunrise — the period when there is little or no sunlight, either direct, or diffuse from the sky. But what, exactly, is the reason for this chilling effect?
Why Was the Cavity Colder than the Outside Air Temperature?
The temperature of virtually anything is the result of a balance between (1) energy gained and (2) energy lost. As long as the energy gained exceeds that lost, the temperature will rise. This was clearly seen when I closed up The Box, and the rate of sunlight absorption in the cavity exceeded the rate of energy lost by infrared emission (and any — hopefully small — conductive losses). The temperature skyrocketed.
But once the rate of energy loss exceeds that gained, then the temperature will fall, as was seen when The Box entered the shade. Then, then rate of IR energy lost (which increases rapidly with temperature) exceeded that gained from diffuse solar radiation, and the cavity temperature fell.
So, at night when there is no solar energy available, what is to prevent the cavity from getting very cold? Outer space is supposed to emit near absolute zero, 3 K. The Box’s cavity enters the hours of darkness at something like 300 K temperature. At 300 K, and assuming an IR emissivity of 0.99, the cavity is emitting IR at a rate of just over 400 Watts per sq. meter. Assuming the box is very well insulated, and is not leaking air, what is to prevent the cavity temperature from dropping well below freezing (273 K)?
The answer is downwelling IR from the sky. During the day in the summer, the broadband infrared sky temperatures viewed from the ground generally runs about 10 – 20 deg. F cooler than the near-surface air temperatures. This source of energy must exist, because without it the temperature of a cavity in a well insulated box at night would plunge even faster than we saw it heat up when exposed to indirect sunlight. And that rapid rate of temperature rise was due to only about 2 Watts per sq. meter! Imagine what in imbalance of 400 Watts per sq. meter would do.
Instead, the sky emits at only a slightly lower temperature than the surface, so the cavity cools only a little at night: about 4 deg. F cooling out of a “potential cooling” of 15 deg. F, assuming the IR emissivity of the cavity is 1.0.
By the way, I calculate that, if the cavity emissivity was only 0.90 rather than the advertised 0.99 (we really don’t know), we could explain the entire 4 deg. F drop based upon the cavity coming to a radiating temperature equal to that of the sky.
Presumably, once drier air arrives here in Alabama in another couple months, I should see larger temperature falls in the cavity, since water vapor is the Earth’s main greenhouse gas. In the meantime, I’m open to suggestions regarding simple ways to make The Box more efficient at rejecting all sources of energy except downwelling infrared radiation from the sky.
…a radiation source which some say, does not exist. 😉
 |

 Home/Blog
Home/Blog



