Probably as the result of my recent post explaining in simple terms my “skepticism” about global warming being mostly caused by carbon dioxide emissions, I’m getting a lot of e-mail traffic from some nice folks who are trying to convince me that the physics of the so-called Greenhouse Effect are not physically possible.
More specifically, that adding CO2 to the atmosphere is not physically capable of causing warming.
These arguments usually involve claims that “back radiation” can not flow from the cooler upper layers of the atmosphere to the warmer lower layers. This back radiation is a critical component of the theoretical explanation for the Greenhouse Effect.
Sometimes the Second Law of Thermodynamics, or Kirchoff’s Law of Thermal Radiation, are invoked in these arguments against back radiation and the greenhouse effect.
One of the more common statements is, “How can a cooler atmospheric layer possibly heat a warmer atmospheric layer below it?” The person asking the question obviously thinks the hypothetical case represented by their question is so ridiculous that no one could disagree with them.
Well, I’m going to go ahead and say it: THE PRESENCE OF COOLER OBJECTS CAN, AND DO, CAUSE WARMER OBJECTS TO GET EVEN HOTTER.
In fact, this is happening all around us, all the time. The reason why we might be confused by the apparent incongruity of the statement is that we don’t spend enough time thinking about why the temperature of something is what it is.
How Cooler Objects Make Warmer Objects Even Hotter
One way to demonstrate the concept is with the following thought experiment, which I will model roughly after the Earth suspended in the cold of outer space. Even my oldest daughter, a realtor who has an aversion to things scientific, got the right answer when I used this example on her.
Imagine a heated plate in a cooled vacuum chamber, as in the first illustration, below. These chambers are used to test instruments and satellites that will be flown in space. Let’s heat the plate continuously with electricity. The plate can lose energy only through infrared (heat) radiation emitted toward the colder walls of the chamber, since there is no air in the vacuum chamber to conduct the heat away from the plate. (Similarly, there is no air in outer space to conduct heat away from the Earth in the face of solar heating.)
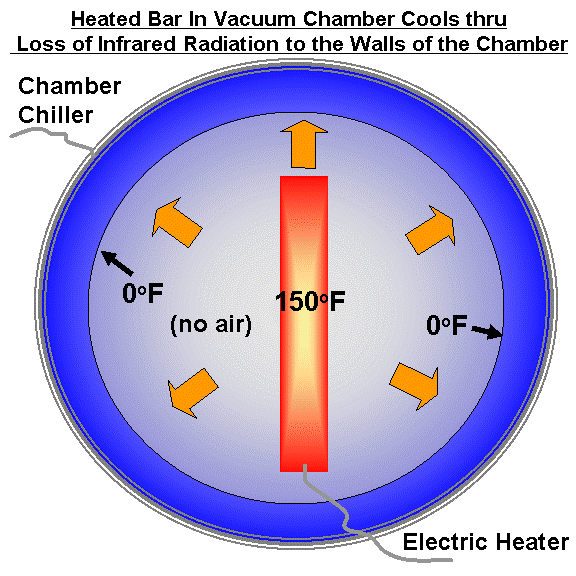
The plate will eventually reach a constant temperature (let’s say 150 deg. F.) where the rate of energy gain by the plate from electricity equals the rate of energy loss by infrared radiation to the cooled chamber walls.
Now, let’s put a second plate next to the first plate. The second plate will begin to warm in response to the infrared energy being emitted by the heated plate. Eventually the second plate will also reach a state of equilibrium, where its average temperature (let’s say 100 deg. F) stays constant with time. This is shown in the next illustration:
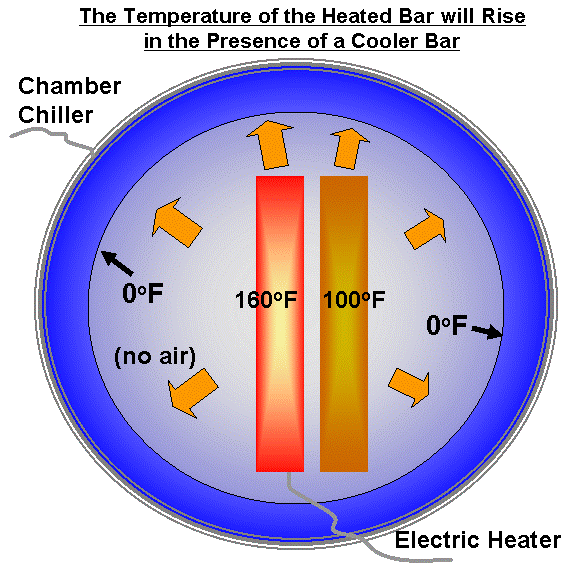
But what will happen to the temperature of the heated plate in the process? It will end up even hotter than it was before the cooler plate was placed next to it. This is because the second plate reduced the rate at which the first plate was losing energy.
(If you are unconvinced of this, then imagine that the second plate completely surrounds the heated plate. Will the heated plate remain at 150 deg., and not warm at all?)
Since the temperature of an object is a function of both energy gain AND energy loss, the temperature of the plate (or anything else) can be raised in 2 basic ways: (1) increase the rate of energy gain, or (2) decrease the rate of energy loss. The temperature of everything is determined by energy flows in and out, and one needs to know both to determine whether the temperature will go up or down. This is a consequence of the 1st Law of Thermodynamics involving conservation of energy.
Note that the above example involving 2 plates, one hotter than the other, is apparently where the greenhouse effect deniers (sorry, I couldn’t help myself) would claim the “physically impossible” has occurred: The presence of a colder object has caused a warmer object to become even hotter. Again, the reason the heated plate became even hotter is that the second plate has, in effect, “insulated” the first plate from its cold surroundings, keeping it warmer than if the second plate was not there.
The only way I know of to explain this is that it isn’t just the heated plate that is emitting IR energy, but also the second plate….as well as the cold walls of the vacuum chamber. The following illustration zooms in on the plates from our previous illustration:
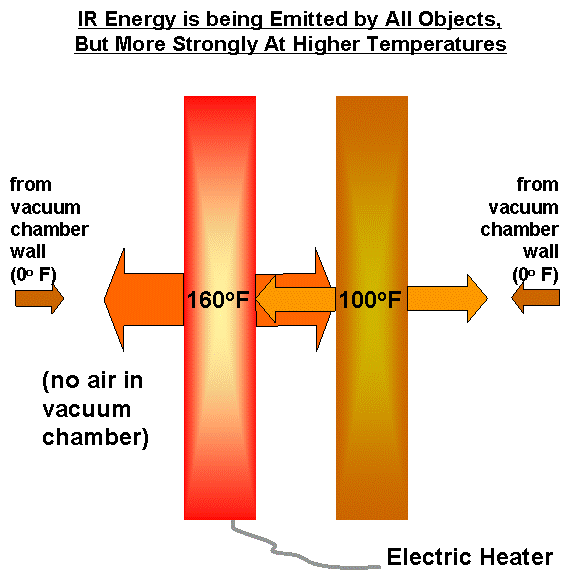
What happens is that the second plate is heated by IR radiation being emitted by the first plate, raising its temperature. The second plate, in turn, cannot cool to the temperature of the vacuum chamber walls (0 deg. F) because it is not in direct contact with the refrigerant being used…it can only lose IR at a rate which increases with temperature, so it achieves some intermediate temperature.
Meanwhile, the cooler plate is emitting more radiation toward the hot plate than the cold walls of the vacuum chamber would have emitted. This changes the energy budget of the hot plate: despite a constant flow of energy into the plate from the electric heater, it has now lost some of its ability to cool through IR radiation. Its temperature then rises until it, once again, is emitting IR radiation at the same rate as it is receiving energy from its surroundings (and the electric heater).
As we will see, below, in the case of the Earth being heated by the sun, the vacuum chamber “wall” (outer space) is close to absolute zero in temperature. Putting anything between that (essentially infinite) heat sink and the Earth’s surface will cause the surface to warm.
Examples are All Around Us
Examples of objects with lower temperatures causing objects with higher temperatures to become even higher still are all around us.
For instance, in terms of these most basic heating and cooling concepts (energy gain and energy loss), the same thing happens when you put a blanket over yourself when it is cold. The blanket stays cooler than your skin, but it nevertheless makes your skin warmer than if the cooler blanket was not there. Even though the direction of flow of heat never changes (it is always from warmer to cooler objects), a cooler object can still make a warm object even hotter.
It doesn’t matter what the mechanisms of energy transfer are….if the presence of a cooler object keeps a warmer object from losing energy as rapidly as before, the warm object will become even hotter.
But if you insist on another real-world example involving infrared radiation, rather than heat conduction, let’s use clouds at night. Almost everyone has experienced the fact that cloudy nights tend to be warmer than clear nights.
The most dramatic effect I’ve seen of this is in the winter, on a cold clear night with snow cover. The temperature will drop rapidly. But if a cloud layer moves in, the temperature will either stop dropping, or even warm dramatically.
This warming occurs because the cloud radiates much more IR energy downward than does a clear, dry atmosphere. This changes the energy budget of the surface dramatically, often causing warming — even though the cloud is usually at a lower temperature than the ground is. Even high altitude cirrus clouds at a temperature well below than of the surface, can cause warming.
So, once again, we see that the presence of a colder object can cause a warmer object to become warmer still.
Extending the Concept to the Atmosphere
As mentioned above, in the case of the cold depths of outer space surrounding the Earth’s solar-heated surface, ANY infrared absorber that gets between the Earth’s surface and space will cause the surface to warm.
This radiative insulating function occurs in the atmosphere because of the presence of greenhouse gases, that is, gases that absorb and emit significant amounts of infrared energy…(mostly water vapor, CO2, and methane). Clouds also contribute to the Greenhouse Effect.
Kirchoff’s Law of thermal radiation says (roughly), that a good infrared absorber is an equally good infrared emitter. So, each layer of the atmosphere is continuously absorbing IR, as well as emitting it. This is what makes the Greenhouse Effect so much more difficult to understand conceptually than solar heating of the Earth. While the sun is a single source, and most of the energy absorbed by the Earth is at a single level (the surface of the ground), in the case of infrared energy, every layer becomes both as source of energy and an absorber of energy.
It also helps that our eyes are much more sensitive to solar radiation than they (or even our skin) are to infrared radiation. It’s more difficult to conceptualize that which you can’t see.
Our intuition begins to fail us when presented with this complexity. The following illustration shows some of these energy flows: just the IR being emitted upward and downward by different atmospheric layers. If I included arrows representing the IR energy being absorbed by those layers, too, it would become hopelessly indecipherable.
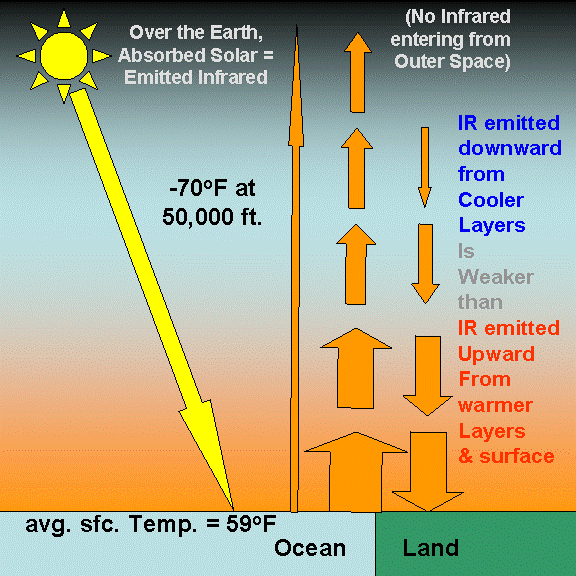
As a result of the atmosphere’s ability to radiatively insulate the Earth’s surface from losing infrared energy directly to the “cold” depths of outer space, the surface warms to a higher average temperature than it would have if the atmosphere was not there. The no-atmosphere, global average surface temperature has been theoretically calculated to be around 0 deg. F.
This, then, constitutes the basic mechanism of the Greenhouse Effect. Greenhouse gases represent a “radiative blanket” that keeps the Earth’s surface warmer than it would otherwise be without those gases present.
In fact, research published in the 1960s showed that, if the current atmosphere suddenly became still – with no wind, evaporation, and convective overturning transporting excess energy from the surface to the upper atmosphere – the average surface temperature of the Earth would warm dramatically, from 0 deg. F with no greenhouse gases, to about 140 deg. F. That the real world temperature is much lower, around 59 deg. F, is due to the cooling effects of weather transporting heat from the surface to the upper atmosphere through convective air currents.
Weather as we know it would not even exist without the greenhouse effect continuously destabilizing the vertical temperature profile of the atmosphere. Vertical air currents associated with weather act to stabilize the atmospheric temperature profile, but it is the greenhouse effect that keeps the process going by warming the lower atmosphere, and cooling the upper atmosphere, to the point where convection must occur.
What About Kirchoff’s Law?
One of the statements of Kirchoff’s Law is:
At thermal equilibrium, the emissivity of a body (or surface) equals its absorptivity.
Many well-meaning people think that one of the consequences of Kirchoffs Law of radiation is that an individual layer of the atmosphere that absorbs infrared energy at a certain rate must also emit energy at the same rate. This is NOT true.
The rate of emission becoming the same as the rate of absorption occurs in the very special case where (1) the temperature has reached thermal equilibrium, and (2) that equilibrium is the result of only those two radiative flows, in and out of the object.
Interestingly, this condition of a layer emitting the same amount of IR as it is absorbing is virtually never met anywhere in the atmosphere. This is because of the vertical, convective flows which are also transporting energy between layers.
In the global average, air below about 5,000 feet in altitude is absorbing more infrared energy than it emits, while air above that altitude (up to the top of the troposphere, the 80% of the atmosphere where weather occurs) is losing infrared energy faster than it is gained.
The reason why these two regions stay at roughly a constant temperature, despite very different rates of infrared loss and gain, is convective heat transport by weather: air heated by sunlight absorbed at the Earth’s surface has its excess energy transported to the upper troposphere, where a lack of water vapor (Earth’s main greenhouse gas) allows that energy to escape more rapidly to space.
The 2nd Law of Thermodynamics: Can Energy “Flow Uphill”?
In the case of radiation, the answer to that question is, “yes”. While heat conduction by an object always flows from hotter to colder, in the case of thermal radiation a cooler object does not check what the temperature of its surroundings is before sending out infrared energy. It sends it out anyway, no matter whether its surroundings are cooler or hotter.
Yes, thermal conduction involves energy flow in only one direction. But radiation flow involves energy flow in both directions.
Of course, in the context of the 2nd Law of Thermodynamics, both radiation and conduction processes are the same in the sense at the NET flow of energy is always “downhill”, from warmer temperatures to cooler temperatures.
But, if ANY flow of energy “uphill” is totally repulsive to you, maybe you can just think of the flow of IR energy being in only one direction, but with it’s magnitude being related to the relative temperature difference between the two objects. The result will still be the same: The presence of a cooler object can STILL cause a warmer object to become even hotter.
Anyway, that’s my story, and I’m sticking to it. Until someone convinces me otherwise.
So, let the flaming begin! No, really, have fun…but if you want your comments to remain available for others to read, please keep it civil.
 |
SUGGESTION FROM ROY (7:50 a.m. Monday, July 26): If you want to add intelligently to this discussion, you need to actually read (1) what I have said, and (2) what others have said. Chances are, your point has already been made and discussed.

 Home/Blog
Home/Blog



