I continue to receive a steady stream of comments and e-mails which challenge my views on how the climate system operates. And that’s fine.
I continue to receive a steady stream of comments and e-mails which challenge my views on how the climate system operates. And that’s fine.
But I also seem to get the same questions, over and over. I understand that it is time consuming to wade through all of the blog posts to find the ones where I address certain issues, so from time to time it seems useful to explain (once again) my understanding of things.
So, here’s my latest attempt at explaining temperature change, the “greenhouse effect” (yes, I know it’s not like a real greenhouse, but thanks for the tip), and why alternative theories cannot yet replace greenhouse theory.
Temperature is the Result of Energy Gain AND Energy Loss
The temperature of something (the Earth’s surface, an atmospheric layer, the human body, a pot of water on the stove) is related to rates of energy gain and energy loss. Thus, generally speaking, you can increase temperature in one of two ways: (1) increase the rate of energy gain, or (2) decrease the rate of energy loss. (There can also be conversion between energy types, of course, but for simplicity here I am just talking about thermal energy, that is, sensible heat.)
For example, if you are lying in bed and are too cold, you can turn up the electric blanket (increase energy gain), or add a regular blanket (decrease the rate of energy loss). If you add even more blankets, you will gradually make the temperature under the blankets higher…but at the same time you make the temperature of the outer blankets colder.
Heat will always flow from higher temperature to lower temperature, but this does not mean that temperatures cannot change. The actual temperatures at different depths in the blankets depend upon the rate of energy gain and energy loss at those different depths, not just the rate of energy input into the system.
The same is true of the climate system, and when explaining the surface temperature of the Earth. It does not matter whether we are talking about radiative heat transfer, or conduction, temperature is a matter of energy gain versus energy loss.
The Greenhouse Effect Decreases the Rate of Energy Loss by the Earth’s Surface
The atmospheric gases most responsible for IR absorption and emission in the atmosphere (“greenhouse gases”) act like a radiative blanket, cooling the middle and upper layers, but warming the lowest layers and the surface.
This leads to two common misconceptions on the part of those who believe the greenhouse effect does not exist:
FIRST, contrary to the assertions of some, the rate of IR absorption and emission of atmospheric layers are, in general, NOT the same. While the rate of IR absorption does not change much with the temperature of the absorber, the rate of IR emission increases rapidly with temperature.
For example, if you took a sample of carbon dioxide gas at a very cold temperature and then irradiated it with infrared light, it would warm. (Some seem to believe its temperature would not change). The warming results because the gas would (initially) be absorbing more IR than it emits. But as it warmed, it would emit IR at a progressively greater rate. If this was the only process of energy gain/loss (IR absorption and emission), then the gas would warm until it reached a temperature where the rate of IR emission equaled the rate of IR absorption. It would end up having a higher temperature than it had before it was heated with IR radiation.
The SECOND misconception is that because greenhouse gases allow the atmosphere to cool to outer space, adding more GHGs can’t cause warming. While it is true that GHGs do lead to an overall decrease in the mass-weighted average temperature of the atmosphere, their altering of the energy budget of individual layers leads to net warming of the lowest layers of the atmosphere.
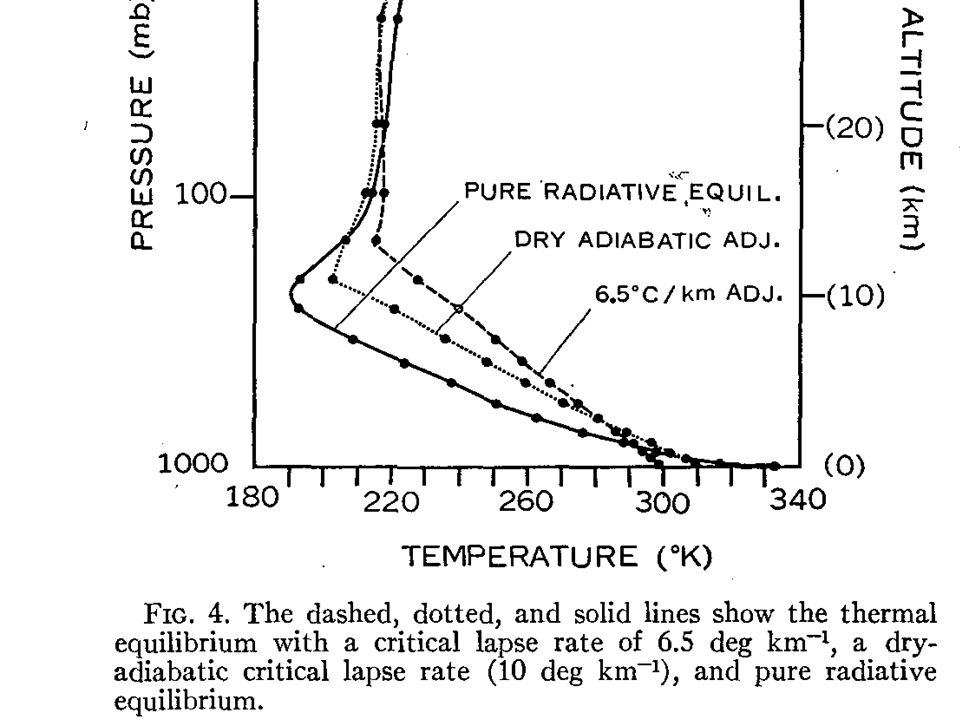
One of the first theoretical demonstrations of this was by Manabe and Strickler (1964), Fig. 4 from which I have reproduced below.
In the case of pure radiative equilibrium (no atmospheric convection, and each layer comes into radiative equilibrium), the surface and lowest atmospheric layers become very hot, while the middle and upper layers become very cold (recall my example of adding blankets over your body?)
In the real world, the atmosphere cannot support such an extreme temperature contrast, which is convectively unstable, and so heat is transported from lower layers to higher layers through convective transport (also seen in the above figure with 2 different assumed temperature lapse rates).
Since most of what we observe as “weather” is the result of convective overturning of the atmosphere, it is actually the greenhouse effect which creates weather. If the atmosphere did not absorb and emit IR energy, it would probably come to the same temperature as the Earth’s surface (an isothermal atmosphere). This would cause all atmospheric convection to cease. (I have yet to test this with a model…the vertical heat transfer from the surface would be through conduction alone, which is very inefficient in air since it is such a good insulator, and which would take a very long time to equilibrate).
By the way, if you are wondering why the stratosphere (above about 200 mb in the illustration) is so different, it is because there is a an additional radiative energy source there: solar ultraviolet energy absorption by ozone.
The Issue of “Back Radiation”
In the usual explanation of the greenhouse effect, one component of the vertical energy flows is downwelling IR radiation from the atmosphere to the surface. The existence of this “back radiation” is disputed by some people because of two seemingly counter-intuitive features:
(1) the back radiation has an average value that is even larger than the energy source for the climate system, solar radiation, which would seem to violate the 1st Law of Thermodynamics; and
(2) it flows from lower to higher temperatures, seemingly in contradiction to the 2nd Law of Thermodynamics.
But the same objections could be made against many systems which create very high temperatures. You can pump energy into a system at a certain rate, and insulate the system so that it cannot lose heat easily and thus increase temperatures to very high levels. This is because, as was discussed at the beginning of this article, the temperature of an object is related to energy gain (input) and energy loss (output)– not to energy gain alone.
So, if you continuously pump a certain number of Watts per sq. meter into a highly insulated system, the interior temperature can rise so high that the resulting infrared emission at the high temperature in Watts per sq. meter exceeds the rate of energy input into the system.
But no physical laws are violated because there are energy flows both outward AND inward at those high temperatures, and it is only the net outward flow which cannot exceed the input into the system.
A Back Radiation Thought Experiment
If you still have an aversion to the idea of back radiation, flowing against the net flow of radiation in the opposite direction, then explain to me what happens in the following example.
Imagine two plates at two different temperatures facing one another. Let’s say one plate is at 100 deg. C and the other is at 0 deg. C. It doesn’t really matter whether this is in a vacuum, or with air around the plates, the concept still applies.
It’s pretty clear that the hotter plate will lose IR energy to the colder plate at a certain rate (which will decrease over time as the plates gradually equilibrate to the same temperature).
But now imagine that the cooler plate is nearly the same temperature (99 deg. C) as the hotter plate (100 deg. C). It will be obvious to most people that the net flow of IR energy from the 100 deg. C plate to the slightly cooler plate will be at a slower rate than it was before.
But why should that be? In both cases the 100 deg. C plate is emitting IR at the same rate, yet the NET flow of IR is reduced if the cooler plate is not as cold.
The reason is that there is also “back radiation” from the colder plate to the warmer plate, which changes the energy budget (energy gain versus energy loss) of the hotter plate. If you STILL don’t like the idea of back radiation (“back” is admittedly superfluous), then just think in terms of the reduced rate of flow from the warmer plate to the cooler plate when their temperature difference is reduced.
Either way, when you reduce the rate of net energy loss from an object, the object will have a higher temperature than if you didn’t reduce the rate of energy loss.
Concluding Remarks
The effect of infrared radiation on the average temperature profile of the atmosphere is complex, and is difficult to comprehend based upon intuition alone. It was not until we developed our own radiative transfer model that we were able to build intuition regarding what happens in a greenhouse atmosphere (with or without convection).
The temperature of the Earth’s surface is an energy budget issue. Even for the same rate of energy input from the sun, the average surface temperature can vary widely depending upon the atmosphere’s ability to restrict the rate of energy loss from the surface, either radiatively or convectively.
“Compressional heating” cannot explain the relative warmth of the Earth’s surface because, if the atmosphere could not cool from IR radiation, it would warm to the same temperature as the surface. This is an isothermal atmosphere, which is convectively stable, and so all convection would stop. Without convection, there is no “adiabatic lapse rate” which describes how a parcel of air warms as it descends from a high altitude to a lower altitude.
Now, it is true that if you took an imaginary mass of air at some uniform temperature floating in outer space and dumped it onto the Earth, compressional heating would initially make the lowest layers warmer. But the final vertical temperature profile that would eventually result would be very different than the initial, as it would depend upon the resulting energy budget in each layer, which in turn would depend upon the abundance of greenhouse gases.
So, what does all of this mean for global warming? Well, exactly how much warming will occur from adding carbon dioxide to the atmosphere remains an open question. The IPCC thinks the sky is the limit. I think there is evidence that it could be quite small.
But while I am in general supportive of questioning even our most cherished and long-held scientific beliefs, I do not yet see a reason for abandoning the basic physics of the greenhouse effect.
In any comments submitted below, please refrain from strawman arguments, or diversionary tactics which divert attention from the central issues addressed above. I will not delete any comments, but I reserve the right to flag any I deem to be intellectually dishonest.

 Home/Blog
Home/Blog