One of the most annoying things about climate forecasts is the apparent need to predict catastrophe.
Of course, it makes good press, like the latest from Bryan Walsh at Time, Climate Change Could Cause the Next Great Famine.
While such theories can always find a home with some learned academics, for those who ‘do’ rather than ‘teach’, the world is a very different place.
For the last 4 years, I have spoken at a Kansas City conference of grain growing and investment interests organized by The ProExporter Network, a company which tracks and predicts both U.S. and international grain markets and growing conditions, especially for corn, soybeans, and wheat.
I was with these folks again last week, and from what I hear, there have been no negative climate-related changes which have been identified. If they do exist, they are swamped by technological improvements…and maybe even the positive effects of CO2 fertilization (which has somewhat conflicting research results for maize).
Here in the U.S, as well as globally, grain production as well as yields (in bushels per acre) have been on an upward linear trend for at least 50 years, primarily due to improvements in varieties (e.g. with greater drought tolerance) and growing practices:
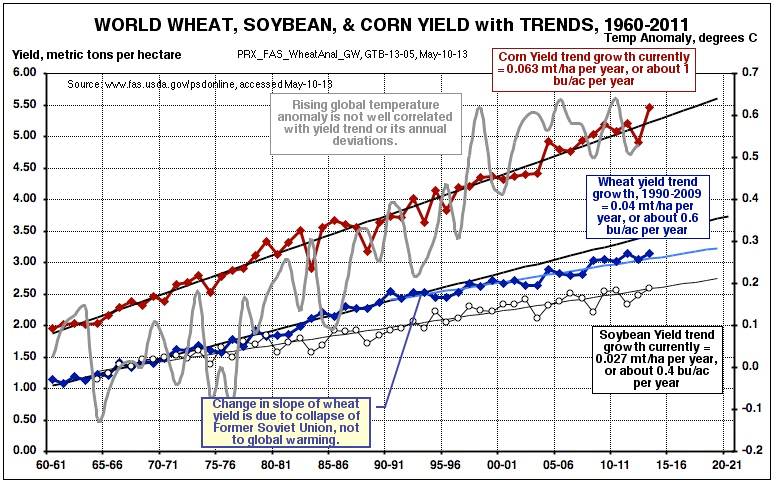
Most year-to-year interruptions in normal growing weather are due to heat waves and droughts, or less frequently, floods. High corn yields are favored by a warm spring with dry planting weather, a not-too-hot summer with sufficient rain (the most important growing period), and a warm, dry fall.
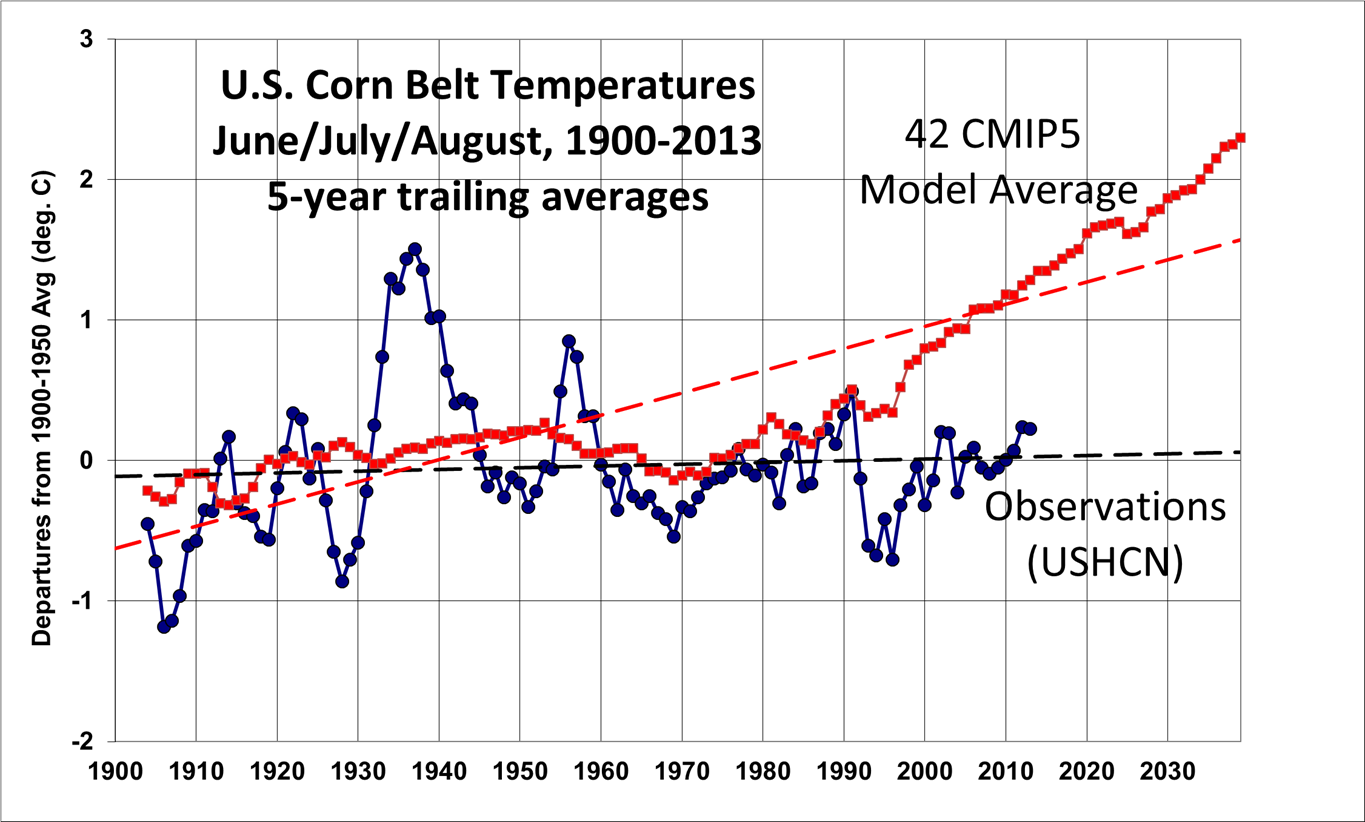
If we examine observed summer (June/July/August) temperatures over the corn belt, we see no obvious warming in the USHCN data. This is in stark
contrast to the average of 42 climate models available through the KNMI Climate Explorer for approximately the same region as the corn belt:
Needless to say, the average model expectation of warming has not materialized in the corn belt. The corresponding average precipitation change in the models (not shown) has a near-zero trend for the corn belt, while there has been maybe a 10% increase in observed precipitation over the last 100 years, largely due to the Dust Bowl days early in the record.
The IPCC claims there is a negative impact of global warming on corn, but the experts I have talked to say there is no way to get that out of the data. You would have to have accurate quantitative knowledge of the technological trend, which you don’t.
In other words, without an accurate removal of the factors leading to the huge increase in corn yield (which is not possible), you can’t back out of the data any kind of climate-related signal. (If anything, the face-value evidence is that warming leads to higher yields, not lower.)
And without that accurate quantitative knowledge (and no evidence of observed corn belt climate change anyway), they tell me there is little reason to depart from a forecast of slowly increasing corn yields in the coming years.
So, unless you are an academic who is trying to remain relevant to the real world by forecasting doom and asking for government grants to support your Malthusian view of the world (wherein population increases exponentially and food production remains more constant), the real world scenario is that population will level off in the next 50 years, while grain production and yields will likely continue to grow, at least for the foreseeable future.

 Home/Blog
Home/Blog