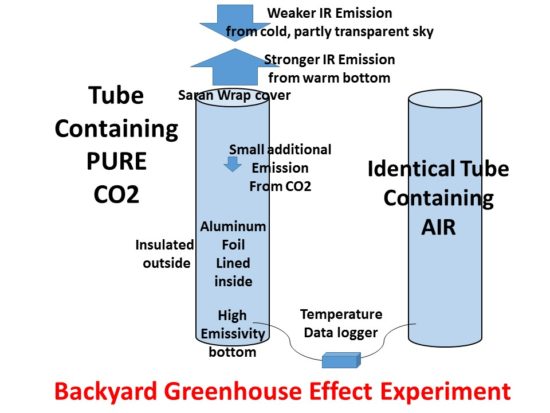
In my continuing battle to keep people from being led astray by bad science, I sometimes try to think of new ways to demonstrate the existence of the Earth’s so-called greenhouse effect (GHE).
I won’t bore you with all of the many evidences I’ve already offered over the years, but instead get right to the experimental setup, which involves putting pure carbon dioxide in one tube, air in a second, identical tube, and measure how much the temperature at the bottom of the tubes cools down during a clear night. I believe that, despite the small path length of CO2, it might be possible to measure a reduction of cooling the the CO2-filled tube versus and identical tube containing air.
I would prefer to have a setup like this where the additional, incremental effect of more CO2 on the atmosphere “partially blocking the view of cold outer space” on an actual near-surface air temperature is measured. More of a real-world demonstration. It would be easier to measure how CO2 (or, say, water vapor) blocks the IR emission from a hot target in a laboratory, but that has already been measured thousands of times, to very high precision at many wavelengths and for many gases.
The widespread claim on the web that a temperature warming effect of CO2 can be measured with CO2 in a jar is totally bogus, and Anthony Watts demonstrated how Bill Nye erred in trying to demonstrate such a thing. You have to (1) have long path lengths, and (2) the CO2 must be “partially blocking” the view that a warm object has of the radiatively “cold” sky. It can’t be done inside.
The trouble with actually measuring a temperature effect from the GHE is that the broadband IR effect (over all wavelengths, not just near absorption lines) of greenhouse gases in the atmosphere really only shows up over long path lengths, so that there is appreciable amounts of greenhouse gas. These long path lengths, combined with the vertical temperature structure of the atmosphere, are necessary for the effect to become appreciable. (Right on CO2 absorption lines, the effect can be large over very short path lengths, but these very narrow bands affect only a tiny fraction of the total IR energy being transferred).
Further complicating any simple experimental setup is that the atmosphere is already pretty opaque to IR radiative transfer, making the practical measurement of a temperature change from adding a little more absorbing gas pretty difficult.
If we were to condense all 400 ppm of CO2 in the atmosphere down to the surface, it would fill a column about 4 meters deep. So, if we could fill a 4 ft tall concrete form tube with pure CO2, I calculate that would be about 120 ppm equivalent increase, or 30% increase, in CO2 compared to the total atmosphere.
The question is, would this be enough additional CO2 to change the radiative budget in the tube enough to measurably reduce the nighttime cooling at the bottom of the tube? It might be.
Of course, one could make the tube taller, and so increase the CO2 path length, but the problem is that the bottom of the tube can only see a small portion of the sky (through the top of the tube) to radiatively cool. Thats why the tubes should be lined on the inside with highly IR-reflective aluminum foil, which has IR reflectivity of 0.95-0.97. This will help the high-emissivity bottom of the tube see more of the cold sky, although multiple reflections off the walls might be required.
How might one fill the tube with CO2? Other than purchasing a cylinder and getting it filled from a supplier, one easy way might be to put a block of dry ice (solid CO2) in the bottom and just wait for it to sublimate. The cold gas will also tend to settle in the bottom, and push air out the top, maybe through a pinhole in the highly transparent (>0.9 transparency) plastic wrap cover. I calculate one 1 ft x 4 ft tube has about 0.1 cubic meter volume, which would require about 0.5 lb of dry ice to fill with CO2.
One issue is that CO2 has somewhat less thermal conductivity than air. But if you look up the numbers, you will find that the thermal conductivies involved are so tiny that they can be ignored in the energy budget of the air in the tubes. Air and CO2 are extremely poor conductors of heat.
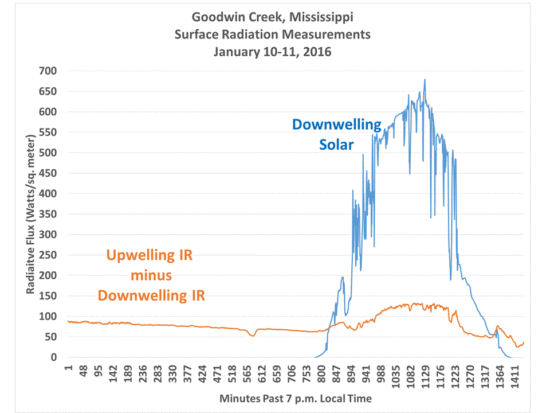
I probably won’t attempt this till fall or winter, when air mass humidity goes down. From monitoring the Goodwin, MS SURFRAD site, I’ve noticed that the difference between upwelling IR and downwelling IR increases from only about 40 W/m2 in the summer to over 80 W/m2 on cold dry days in the winter:
One would need as big a cooling potential as possible so see the effect of adding CO2 in the tube, and that difference between upwelling IR and downwelling IR is what is important for the experiment to work. When that difference approaches zero, say with dense low cloud cover, there will be no radiatively-induced temperature change anyway.
Good insulation is absolutely necessary so that the effect of the surrounding air temperature has minimal impact on the results and the differences in tube interior temperatures will be dominated by IR radiation transfer. Insulation results in a temperature drop below ambient temperature. I have blogged previously about producing 4 deg. F temperature drops below ambient in a Styrofoam box covered with plastic wrap, and have since achieved up to 8 deg. F drop in a standard Styrofoam cooler. The effect shows up strongly within an hour or so of sunset.
The experiment would probably need to be repeated with the tubes swapped since they cannot be constructed exactly the same.
Suggestions are welcome. I’m only going to attempt things that are easy, cheap, and not time consuming.
Finally, no matter how accurately the temperature effect is that is measured (if any) it can’t be used to quantitatively estimate the effect of adding CO2 to the atmosphere. There are too many differences between the experiment and what happens when the extra CO2 is spread out through the full depth of the atmosphere, AND the atmosphere has a chance to respond through changes in clouds, evaporation, precipitation, etc. The simple experiment is meant to simply demonstrate that the GHE exists…that is, that atmospheric greenhouse gases cause a warming tendency of surface temperatures.
(Edited for clarity).

 Home/Blog
Home/Blog